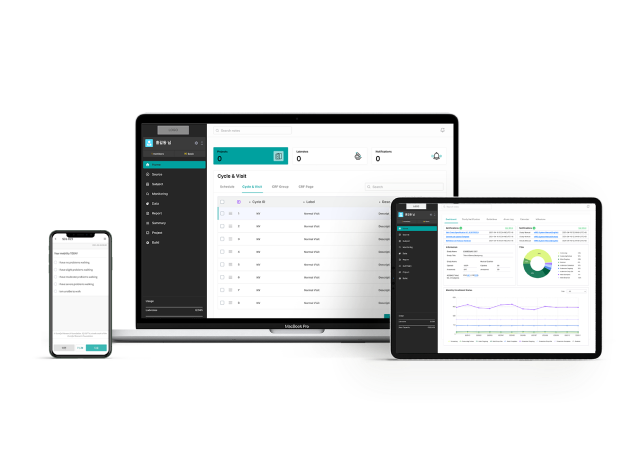
eClint® WEB PORTAL
Results Available Wherever You Are
eClint® is a suite of data and technology enabling solutions designed to increase drug and medical device development efficiency, reduce timelines, improve compliance and drive commercialization for clinical trial development.